- +44 800 052 2424 +44 800 052 2425
- info@ukglobal.uk www.ukglobal.uk
ISO 15378:2015 CERTIFICATION ISO 15378:2015 identify necessities for a quality management system where an organization requirements to demonstrate its capability to give primary packaging materials for medicinal products, which constantly meet client necessities, including regulatory necessities and International Standards applicable to primary packaging materials. |
BENEFITS OF ISO 15378:2015 CERTIFICATION
|
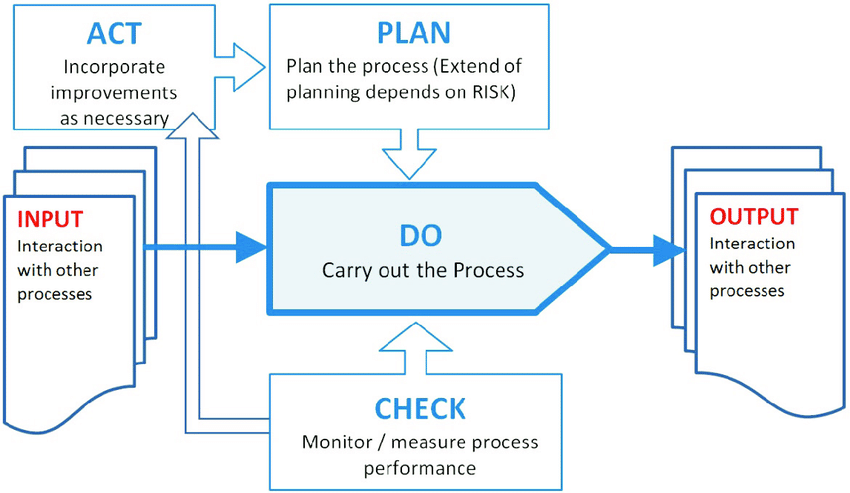
Structure Introduction How to get / what is Process for ISO certification? To get the ISO certification, please fill up below given form & our team will contact you. Step-1 Query from the Client Step-2 Filling up the Application form Step-3 Agreement Approval Step-4 Stage-1 Audit Step-5 Stage-1 Audit NC’s Closing Step-6 Stage-2 Audit Step-7 Stage-2 Audit NC’s Closing Step-8 Certificate Release
|
 |